服务热线
搜题▪组卷
| 温度/℃ | 10 | 20 | 40 | 60 |
| 溶解度/g | 21 | 32 | 64 | 110 |
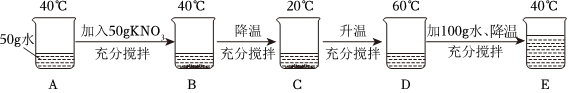

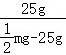 =
=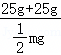 ,m=100。
,m=100。| 温度/℃ | 20 | 32.4 | 40 | 60 | 80 | 100 | |
| 溶解度/g | NaCl | 36.0 | 36.4 | 36.6 | 37.3 | 38.4 | 39.8 |
| Na2SO4 | 19.4 | 52.0 | 48.4 | 45.3 | 43.7 | 42.3 | |

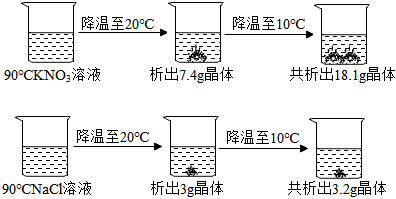
| 温度/℃ | 30 | 50 | 70 | 90 | |
| 溶解度/g | NaCl | 36.3 | 37.0 | 37.8 | 39.0 |
| KNO3 | 45.8 | 85.5 | l38 | 202 | |
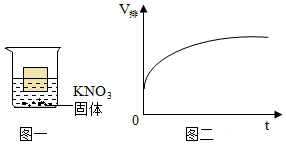
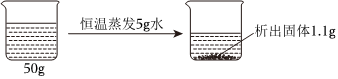
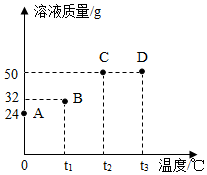 将30g固体物质X(不含结晶水)投入盛有20g水的烧杯中,搅拌,测得溶液的质量分别如图中A、B、C、D点所示。回答下列问题:(1)0℃时,物质X的溶解度是 。
将30g固体物质X(不含结晶水)投入盛有20g水的烧杯中,搅拌,测得溶液的质量分别如图中A、B、C、D点所示。回答下列问题:(1)0℃时,物质X的溶解度是 。| 温度/℃ | 0 | 20 | 40 | 60 | 80 | 100 | |
| 溶解度/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
| KCl | 27.6 | 34.0 | 40.0 | 45.5 | 51.1 | 56.7 | |
| 温度(℃) | 0 | 20 | 40 | 60 | 80 | 100 | |
| 溶解度(g/100g水) | 氯化钠 | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
| 氯化钾 | 27.6 | 34.0 | 40.0 | 45.5 | 51.1 | 56.7 | |
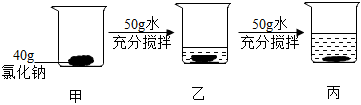
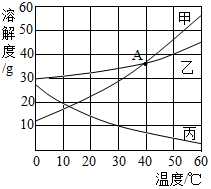 甲、乙、丙三种固体物质(均不含结晶水)的溶解度曲线如图所示。据图回答:
甲、乙、丙三种固体物质(均不含结晶水)的溶解度曲线如图所示。据图回答:| 温度/℃ | 0 | 20 | 40 | 60 | |
| 溶解度/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 |
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | |
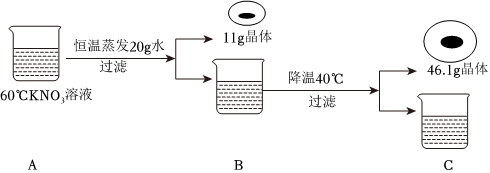
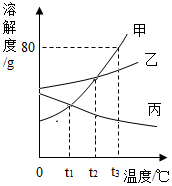 根据如图甲、乙、丙三种固体物质的溶解度曲线,回答下列问题:
根据如图甲、乙、丙三种固体物质的溶解度曲线,回答下列问题: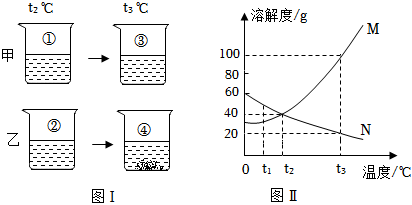
| 温度/℃ | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 溶解度/g | M | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 |
| N | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | |
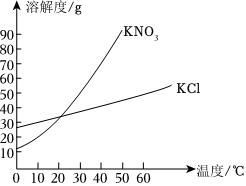

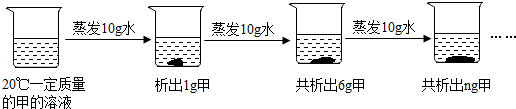
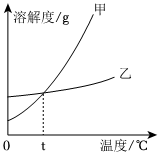 列表法和作图法是常用的数据处理方法。请根据下列图表进行分析。
列表法和作图法是常用的数据处理方法。请根据下列图表进行分析。| 温度/℃ | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 溶解度/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | |
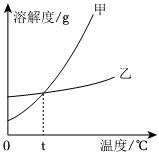 列表法和作图法是常用的数据处理方法。请根据下列图表进行分析。
列表法和作图法是常用的数据处理方法。请根据下列图表进行分析。| 温度/℃ | 0 | 10 | 20 | 30 | 40 | 50 | 60 | |
| 溶解度/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | |

ybw@dyw.com
2024-06-19
初中化学 | | 填空题